Battery second life (5/5)
Second life of batteries, for which the English term “second life” is often used, is a much-discussed topic today. It is also the last topic of our school of batteries. With the growing number of battery applications in the world, there is a growing demand to resolve the question of what traction batteries will be after they have reached their original application. The first projects for the further use of obsolete vehicle batteries are already appearing. But let’s first look at battery technologies and their assumptions about life after life.

Lead and nickel batteries
Lead batteries are among the oldest chemical storage sources. Lead-acid batteries have been known since the 19th century and were invented by Gaston Planté. The positive electrode of a GroE lead-acid battery is even named after him. The construction of lead batteries consists in the use of lead in electrodes and the solution of sulfuric acid is the electrolyte.
The range of lead battery applications is very wide. They are used as backup batteries in UPS systems, in power plants, substations, as starter batteries in cars, on-board batteries in rail vehicles, as storage sources for photovoltaics and also as traction batteries.
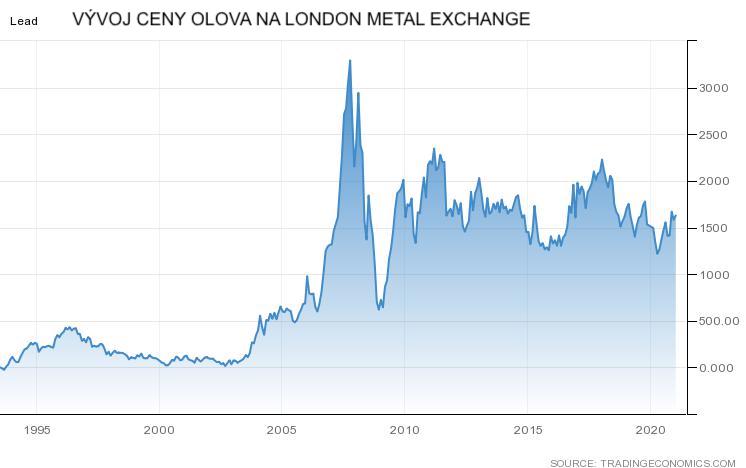
Lead-acid batteries are not suitable for secondary use. Degradation of capacity at the end of life is very sharp and further use is impossible. However, they can be recycled very well and all the material in the old lead batteries is used to produce new ones. Lead, although a toxic element, is very valuable and its price remains relatively stable at 1500 USD / t. The graph in Figure 1 shows the development of lead prices on the London Stock Exchange.
So if you have any old lead-acid batteries, do not hesitate to find the nearest collection point fo secondary raw materials, because you will be well paid for the lead-acid battery as waste.
Nickel cadmium and nickel metal hydride batteries are similar to lead batteries full of metals, which are not exactly friendly to human health. Nickel in larger quantities is a toxic element and in the case of nickel cadmium batteries the harmful effects of cadmium are even worse – therefore their use in the EU has also been significantly reduced.
However, both NiCd and MiMH have the advantage that over 95% of the material can be recovered by recycling to produce new batteries. And where can we find them? Today, NiCd batteries are used in rail applications as on-board batteries, especially in areas with lower temperatures, which handle better than lead batteries. NiMH batteries can be found mainly as rechargeable pencil batteries and in some electronics. However, they are also used in electromobility, especially in older hybrid types.
Lithium batteries
Lithium batteries, unlike the previous two types, are many more separate types that differ in their chemical composition. Nevertheless, most of them contain rare chemical elements, of which there are not enough in the world. Therefore, finding efficient recycling is an important milestone in the path to electrifying not only transport but also other areas of human life. Even today, it is difficult to imagine life without lithium batteries. Our mobile phones, our computers, flashlights and cordless tools and other devices all use lithium batteries. When we include electrified transport, industrial applications such as handling equipment, but also battery storage, there is a large number of batteries, which once awaits the need for their ecological disposal. Until recently, efficient recycling of lithium batteries was the music of the future.
Until now, the recycling process has yielded more secondary components, such as copper from cell jumpers or aluminum from cell bodies, but not precious and rare metals that are part of the battery itself.
Now comes established commercial processes that can get more than 80% of reusable material from lithium batteries. They can also obtain from recycled batteries those substances that could not be obtained before. These are cobalt, magnesium, nickel and lithium. An example is the Canadian company Li-Cycle, which, thanks to its unique cell processing technology and subsequent hydrometallurgical process, is able to recover 95% of battery cell materials for further use. The Finnish energy company Fortum is following the same path, which not only operates power plants, including nuclear power plants, but is also focusing on the process of recycling batteries that have really reached the end of their life.
But what to do with batteries that can still be used?
This topic is becoming very interesting. Even though we are still at a time when electromobility is still developing, we are already starting to decide today what to do with the batteries that we have replaced with new ones in our vehicles.
For example, we don’t have to go far to use discarded batteries. In its second life LTO operation, nano power uses a battery storage with a capacity of 80 kWh (see photos in Figure 2), which was composed of discarded batteries that originally operated in AGV robots in Porsche factories. This battery storage is used to measure the capacities of traction and other batteries. Energy is transferred from the second life of the battery storage to another battery and back. Thus, when measuring capacity, the battery does not have to be discharged by a load that is essentially a powerful heater, and energy is thus wasted in the heat produced during discharging. After discharging, nano power does not have to buy energy from the mains to recharge the battery. This concept not only saves costs, but also cleverly uses batteries discarded from the original application.
Another interesting concept of nano power for the use of discarded batteries is the charging of electric cars, electric buses and other electric vehicles.
As has been said several times, traction batteries cease to be effective for use on vehicles after their capacity has dropped to 80% of its original value. However, LTO batteries in particular, which have been used in power-intensive applications, have previously reached other well-known end-of-life conditions. This is to achieve 150% of the original internal resistance, which then significantly limits the high performance in the order of 8C of the C-Rate indicator, which LTO can handle. (Reminder: C-Rate, ie the coefficient of current carrying capacity, indicates the speed of charging and discharging the battery. For practical use, it represents the ratio of charging and discharging power of the battery in kW and its capacity in kWh.) After many years of operation, the LTO battery is no longer able to reach the C-Rate of 8C, but still has sufficient capacity and especially cyclic life for applications where it will be regularly charged and discharged. Second life LTO is thus an ideal solution to support charging as a source of energy, eg to reduce peak shaving, where the battery supplies the missing power to the appliance – eg a charging station for electric cars.

The future of second life applications
As the first projects that use already discarded batteries show, there are no limits to ideas in this field. The near future promises the possibility of significantly higher use of traction batteries, which are no longer usable for vehicle propulsion, in other interesting projects. Whether it is backup sources, home battery storage, battery systems for energy support, or battery sources reminiscent of the function of a pumped storage power plant, the secondary use of batteries is definitely an area that promises to further develop the accumulation of electricity in batteries.
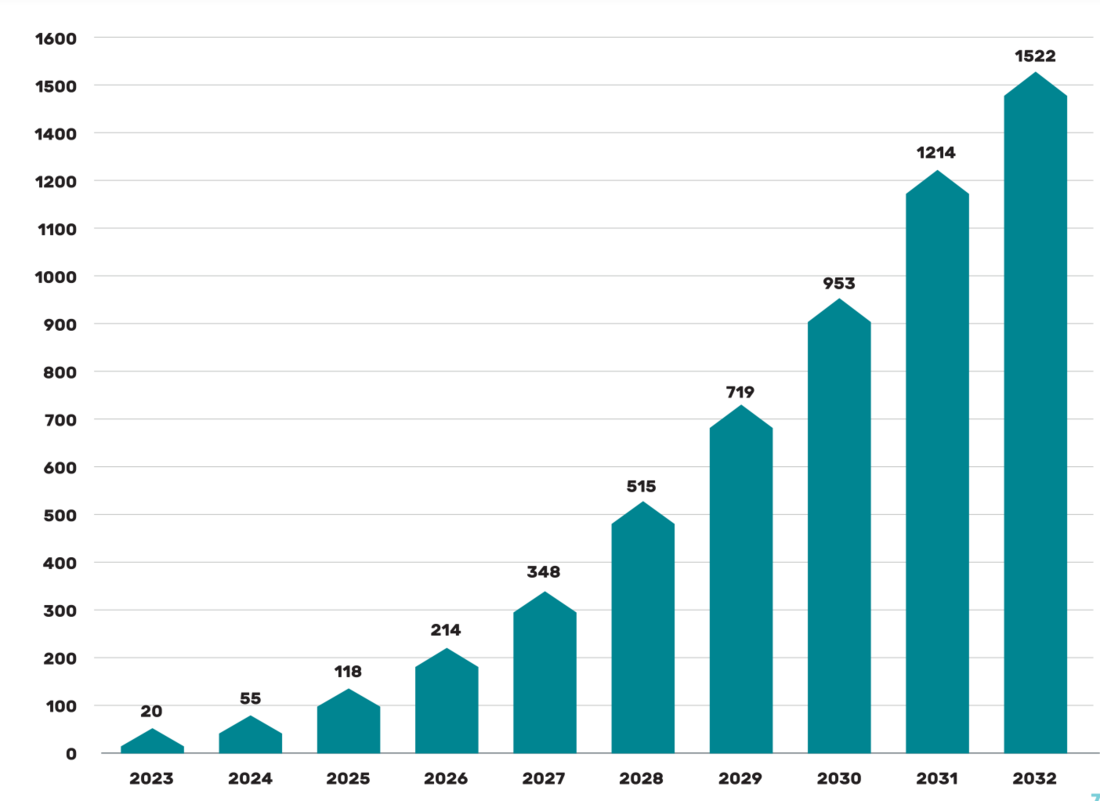
A study by Beryllls from 2018 on the topic of the battery production market mentions the potential for growth in the use of the so-called second life battery sources. From the graph in Picture 3, everyone can get an idea of the expected possibilities of stored energy in the form of secondary lithium batteries.
Summary
This article concludes our short series of Battery School articles. I hope that together we have helped you better understand the basics of choosing the right battery for your application.
Especially for electric road vehicles, batteries make up a very significant part of the acquisition costs. Therefore, it is important to correctly define the key features that a particular application requires from the battery at the time of defining the requirements for tender documents and tenders.
In rail transport, service life is again a key element and the longer, the better, because service costs increase significantly with frequent replacement of traction drive battery sources.
Thank you for taking the time to read this section!
František Šťastný
