Will the future of batteries be solid?
Solid-state batteries and their premiere at electric buses in Wiesbaden
The question in the title of this article is all-encompassing. Battery manufacturers and scientists looking for significant advances in batteries are focusing on so-called solid-state batteries. Solid means strength and rigidity and indicates the form of electrolyte that is used in solid-state batteries. Today, commonly used lithium-ion (Li-Ion) batteries and lithium-polymer (Li-Pol) batteries are known, and now promising solid-state batteries are being added.

But what is the difference and how should solid-state batteries be unique? All the technologies mentioned are lithium batteries. Even solid-state batteries are not a mythical stone for the sages of the battery world. However, it brings major improvements. To understand it, let’s take a closer look at the construction of all three types.
But first, let’s recall the seemingly “familiar and trivial” principle of battery operation: The battery is based on a galvanic cell, which is a chemical source of electrical voltage. It consists of two electrodes – a negative anode and a positive cathode – surrounded by an electrolyte, ie a liquid or solid solution conducting an electric current. The electric voltage is given by the difference of electric potentials on the electrodes, which applies not only to galvanic cells. The electric potential at the electrodes arises in the case of galvanic cells by a chemical reaction between the electrode and the electrolyte. Obviously, the galvanic cell is a source of DC voltage.
The main difference between these types of lithium batteries is in the electrolyte used by these three technologies. Picture 1 shows the main difference between Li-Ion and solid-state batteries. In Li-Ion batteries, which are the most commonly used batteries in electromobility today, the electrolyte is a liquid chemical. In Li-Pol batteries the electrolyte is a polymer gel and in solid-state batteries the electrolyte is solid.
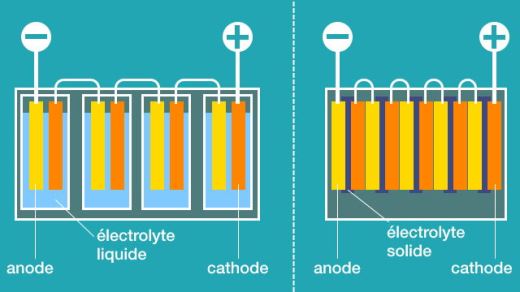
The difference is obvious from the picture. While with Li-Ion batteries and actually also with Li-Pol batteries, it is necessary to connect individual cells into a serial, resp. serial-parallel connections, which are then assembled into modules and those into battery units, with solid-state batteries this can be done inside the battery module and the whole unit. And here’s a major improvement. Less material per cell body means less weight, less volume, and thus significantly better energy density (Wh / l) and specific energy (Wh / kg).
All three types of batteries have the same compound of lithium and other elements, which then declare what type of battery it is and also its other properties (NMC, LFP, NCA, etc.). In Li-Ion, the anode is then carbon, with the exception of LTO, where the carbon anode is replaced by the anode of Li4Ti5O12, or lithium titanium oxide. For Li-Pol batteries, the cathode is the same as for Li-Ion, and this also applies to solid-state batteries. Even Li-Pol batteries bring the advantage of weight. Thanks to their construction, they do not need such a robust cell housing material, and thus bring significant weight savings, which, however, solid-state batteries move even further. Picture 2 shows the design principle of the lithium battery. An electrolyte is shown here, which is separated from both electrodes by separators. With solid-state batteries, a separator is not required, as the separator is itself an electrolyte.
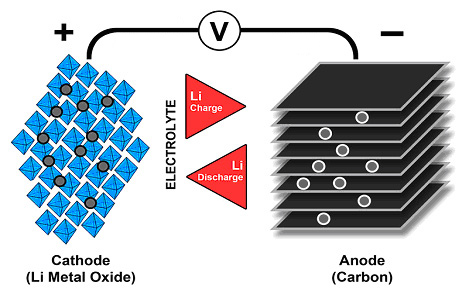
Solid state batteries and their construction thus brings together with significant weight savings, resp. by increasing density and specific energy, as well as other major improvements. That is security. Unlike conventional Li-Ion batteries, it still maintains its shape even when damaged, and thus significantly improves safety in the event of damage, such as an accident. However, solid-state batteries still face their technical limitations. So far, compared to Li-Ion, this is a significantly lower number of cycles, higher internal resistance (and thus higher losses at high power and the impossibility of fast charging) and poor resistance to low temperatures. In contrast, compared to Li-Ion batteries they can contain twice as much energy in the same size and this makes them a candidate for the number one battery of the future.
Solid-state batteries at electric buses in Wiesbaden
The use of solid-state batteries in regular operation on electric buses began in early February 2021 in the German city of Wiesbaden, 280,000. The local carrier ESWE Verkehr has put into operation 21 Mercedes eCitaro electric buses equipped with batteries with this technology.
eCitaro (see photo below) is a 12m long electric bus, in a standard two-door version for 88 passengers. The solid-state batteries supplied for this type of electric bus consist of 6 battery modules with a total capacity of 387 kWh. The batteries are stored on the roof and in the back of the electric bus. The manufacturer also supplies a version with 7 battery modules with a total capacity of 441 kWh, whose transport capacity is 74 passengers, ie about 8% less. In both versions, the number of seats is 29.
Let’s now look at the information from the manufacturer on the relationship between the chemical composition of the batteries and their capacity with respect to the passenger space they allow to use. eCitaro is also supplied with NMC batteries (more about batteries for electric buses and their chemical composition in our Battery School here).
The NMC battery in the two-door eCitaro electric bus with 6 standard battery modules with a total capacity of 146 kWh will allow a transport capacity of 85 passengers. In the variant with 8 standard battery modules with a total capacity of 194 kWh, it will allow a transport capacity of 74 passengers. Let’s compare this with the above parameters of an electric bus with solid-state batteries. We see that, given the transport capacity of the electric bus, the use of solid-state batteries will increase the capacity of the energy storage, and thus the range, per charge, by about 130 – 170% compared to standard NMC batteries. When equipped with an NMC battery with batteries with 33 kWh cells compared to standard NMC batteries with 24 kWh cells, this difference will be about 70 – 100%.

From what has been said above about solid-state batteries, it is clear that this technology is effective to use in night socket charging mode for all-day operation. ESWE Verkehr also follows this path. Therefore, part of the entire Wiesbaden electric bus project is also the charging base in the Gartenfeldstraße garages (see photo below), equipped with intelligent energy management that assigns specific vehicles to specific lines according to the current state of charge of the batteries. It is assumed that with all electricity consumption for own driving and for auxiliary equipment (so-called non-traction consumption), the eCitaro electric bus with solid state batteries in Wiesbaden will cover at least 200 km per charge.

Are there other promising technologies for batteries?
There are. Lithium sulfur, or Li-S, seems to be a very promising technology. The design principle is very similar to Li-Ion batteries, it is actually a lithium ion battery. Lithium Ion batteries typically have graphite anodes. During discharge, lithium ions are released from the anode towards the cathode. The cathode is then usually composed of a compound of lithium and a metal. In Li-S batteries, the Li-S compound replaces the “metal” cathode. This will significantly reduce the weight of the battery.
The specific energy of a Li-S battery can reach up to 550Wh / kg (see Battery University, Future batteries article, published September 8, 2020, available here). Although these batteries have a lower cell voltage (2.1 V), this is offset by a very high specific energy. The counter-argument of this technology is so far a very small number of cycles (about 50 charging cycles), because sulfur is released from the cathode and reacts with the anode. Li-S will probably not become a “game-changer” of accumulation in the near future.
Very interesting numbers are also presented by Lithium-air battery technology. This technology could theoretically be the “holy grail” that scientists are looking for. It can theoretically accumulate much more energy than all current technologies. The researchers tried a similar approach to fuel cells to create a battery that would use access to oxygen. The design is then such that the battery, like the Li-Ion battery, contains an electrolyte, a lithium anode and a catalytic cathode providing a chemical reaction in the battery oxygen. Theoretical specific energy numbers mention values of 13 kWh / kg. That’s fifty times more than current lithium ion batteries with the highest specific energy and just like gasoline. Compared to petrol, however, electric drives with recuperation have minimal losses.
Despite these very promising theoretical numbers, there is no prospect of close commercialization for the time being, as several fundamental technical issues need to be resolved. It is mainly a proposal of a technical solution with an air purifier or a significant improvement in cyclic life. As with other oxygen batteries, the specific power (W / kg), which is very low for these batteries, especially at cold temperatures, also needs to be addressed.
František Šťastný and editorial staff Smartcityvpraxi.cz
Photo © ESWE Verkehrsgesellschaft mbH
